Chapter 10 Student Presentations
10.1 Mortality Crossovers
10.1.1 Outline
10.1.2 Mortality Crossovers: Reality or Bad Data? (Coale and Kisker 1986)
They noticed the following patterns in the age-specific death rates for different countries.
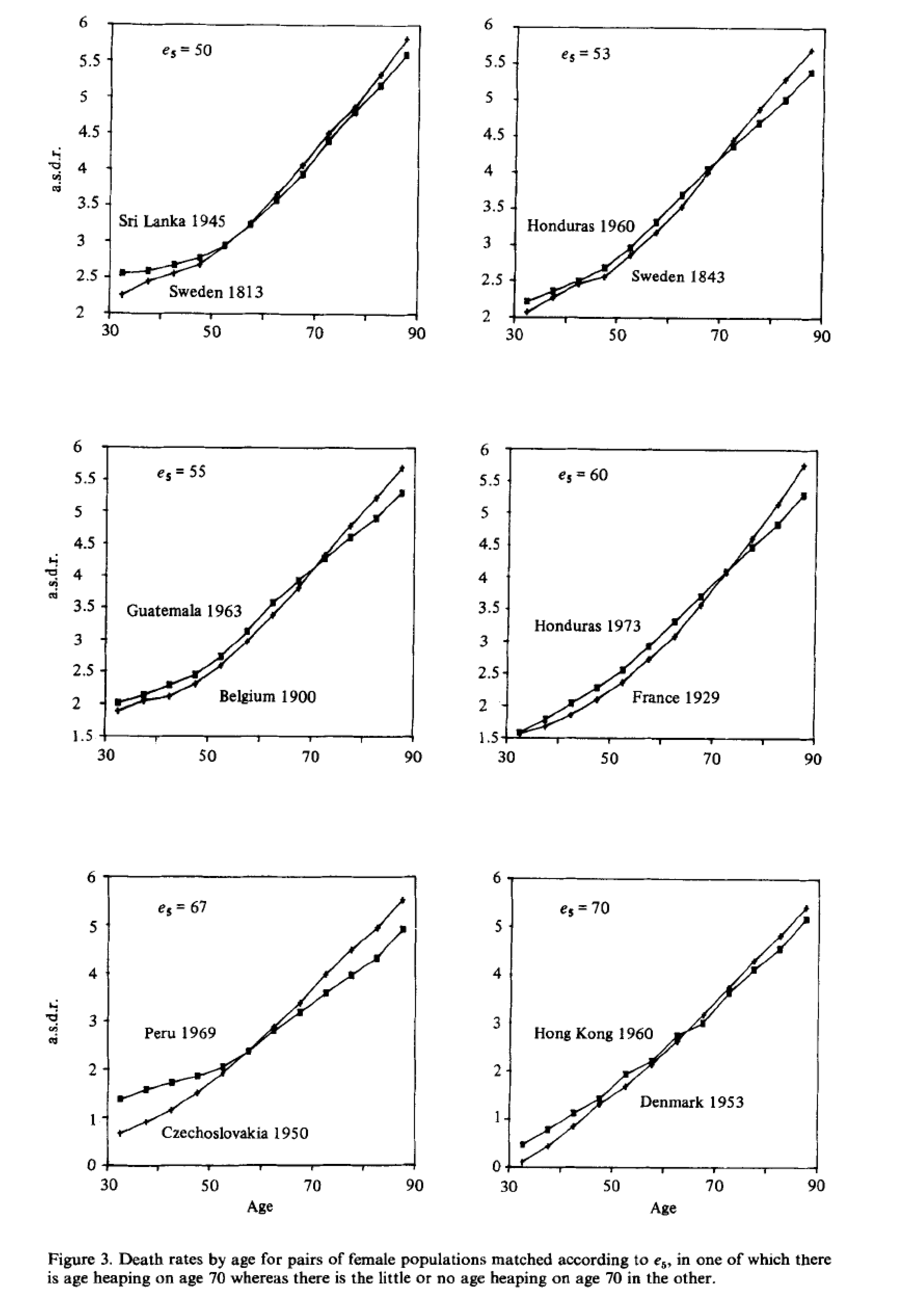
Figure 10.1: Age specific death rates for different countries and cohorts. Source: Coale and Kisker (1986)
- Selection / heterogeneity:
- Elimination of the frailer members of the population at younger ages leaves only the very robust with lower mortality rates.
- Level playing fields at older ages
- Social Security, Medicare, etc.
- Bad data
- Misreporting age of death can lead to biased estimates of mortality rates at older ages
10.1.2.1 Age heaping:
- General pattern of age misstatement, most often rounding up to nearest 5 or 10.
- Begins with a modest upward transfer at age 60 or 70, increases rapidly with age.
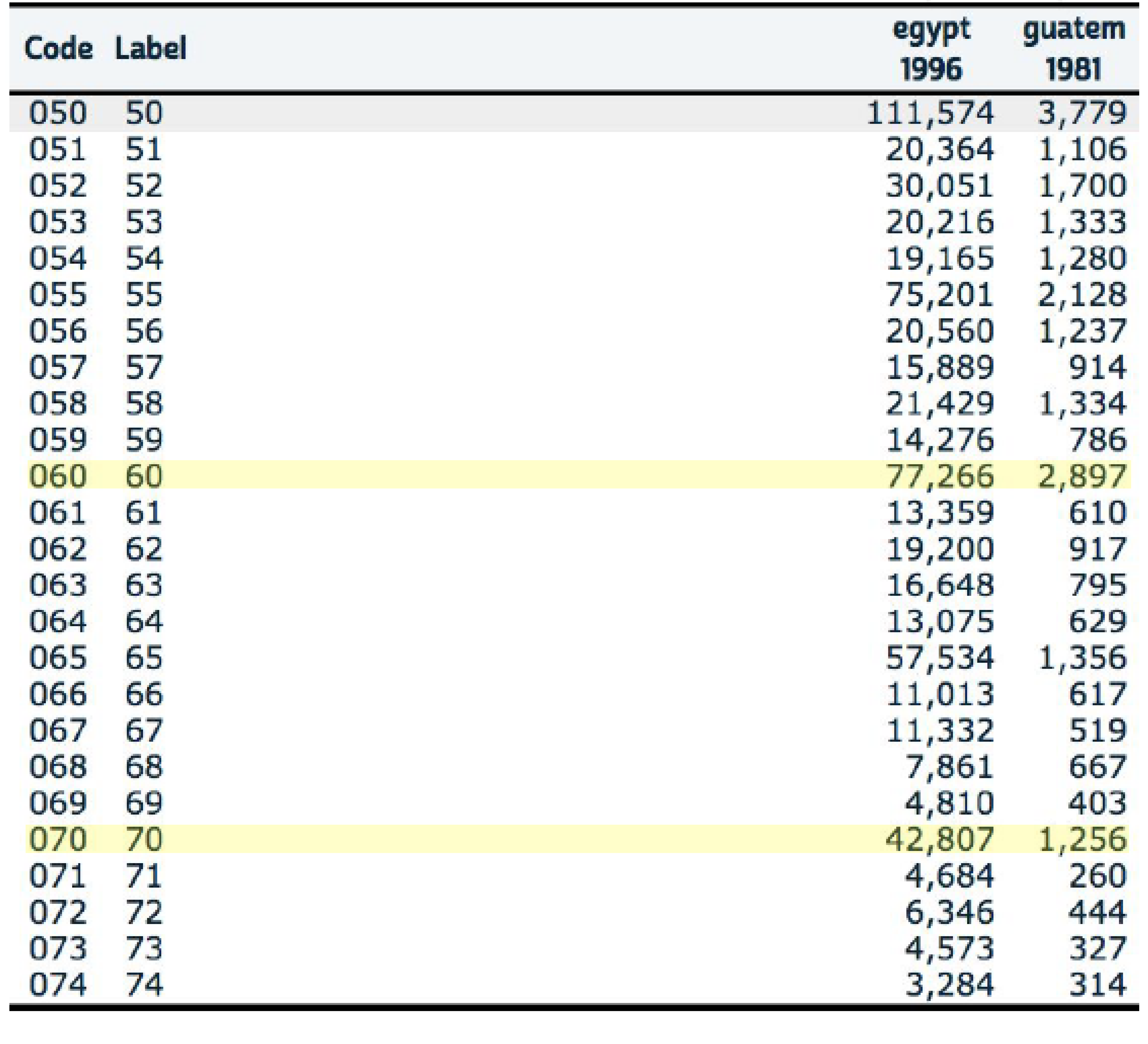
Figure 10.2: Age heaping example Source: IPUMS International
Implications of age heaping:
\(\text{age heaping on age 70} = \frac{Pop_{70}}{(Pop_{69}+Pop_{71})/2}\)
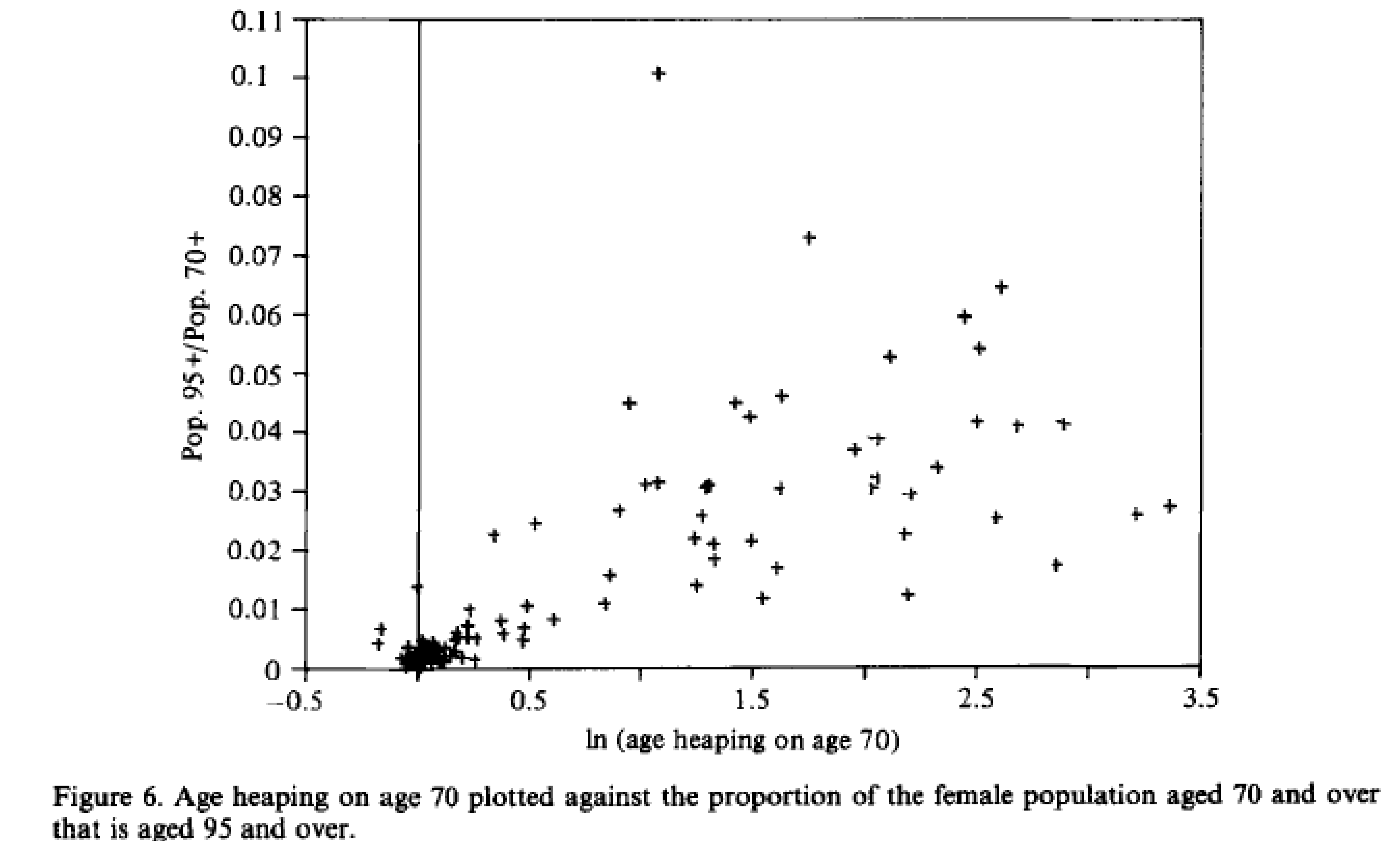
Figure 10.3: Age heaping. Source: Coale and Kisker (1986)
10.1.2.2 Takeaways
- Age overstatement at advanced ages is common and downwardly biases estimates of mortality rates
- Age heaping is associated with age overstatement
- Low quality mortality data can artificially create a mortality crossover
10.1.3 Methods for Evaluating the Heterogeneity of Aging Processes in Human Populations Using Vital Statistics Data: Explaining the Black/White Mortality Crossover by a Model of Mortality Selection (Manton and Stallard 1981)
10.1.3.1 Summary
- A model to compute the ratio of Black and White individual age specific mortality risks (within sex) to determine if the adjustments of heterogeneity and mortality selection is sufficient to remove the crossover.
- Data from the U.S. Black and White populations for the period 1935 to 1975.
- Mortality crossover (Blacks having relatively lower mortality rates) at age 75.
- Could be explained under the proposed model.
- Data quality? Variety of evidence supporting the existence of a crossover.
- Consequently, careful consideration should be made of the population mechanisms by which the crossover might occur.
10.1.3.2 A model of selection
- Life tables are separately calculated for the Black and White populations in the U.S. over the period 1935 to 1975 based upon the assumptions:
- Each population is heterogeneous.
- The initial distribution of individuals in each population is identical (within sex) with respect to variables relevant to longevity. -Individual’s environmental conditions are fixed at birth.
- Operationally, they modified standard life table calculations (Chiang, 1968) to reflect the dependence of mortality rates at advanced ages upon the selection of earlier mortality levels on a heterogeneous population.
10.1.3.3 A little bit of math
- Assumptions:
- The following partial differential equation governs the change of the distribution as cohort age: \[\begin{aligned} \frac{\partial f_{x}(z)}{\partial x}= f_{x}(z)(\bar{\mu}_{x}-\mu_{x}(z)) \end{aligned}\]
- Each person retains the value of \(z\) (longevity characteristics) given at birth.
- Functional forms: \[\begin{aligned} \mu_{x}(z)=z\mu_{x}(1)= z\mu_{x} \end{aligned}\]
- Thus \(z\) may be taken to be a measure of relative (to the standard individual) frailty or “susceptibility to death”. Alternatively, \(1/z\) may be considered as a measure of vitality or “robustness”.
- Variance and frailty relation:
- We also have the following definitions: \[\begin{aligned} \overline{\mu}_x&= \overline{z}\mu_x\\ \overline{z}_x&= \int_0^\infty z f_x(z) dz \end{aligned}\]
- Therefore, we can say that \(\frac{\partial\overline{z}_x}{\partial x}=- \mu_x \sigma_x^2(z)\) \[\begin{aligned} \frac{\partial f_x(z)}{\partial x}&=f_x(z)(\overline{z}\mu_x - z\mu_x)\\ \frac{\partial\overline{z}_x}{\partial x}&= \frac{\partial \int z f_x(z) dz}{\partial x}\\ &= \int z \frac{\partial f_x(z) }{\partial x}dz\\ &= \int z f_x(z)(\overline{z}\mu_x - z\mu_x)dz \\ &= \mu_x \left(\int z f_x(z)\overline{z} dz- \int z^2 f_x(z)dz\right)\\ &= -\mu_x \left(\int z^2 f_x(z)dz - \overline{z} \int z f_x(z) dz \right)\\ &= -\mu_x \left(\int z^2 f_x(z)dz - \overline{z}^2 \right)\\ \frac{\partial\overline{z}_x}{\partial x}&= - \mu_x \sigma_x^2(z) \end{aligned}\]
- This means the “frailer” population members (with high z’s) are being selected earlier than their more “robust” contemporaries (with low z’s)
- Gamma distribution:
- The proportionality assumption has implications for \(f_x(z)\)
- Mortality cannot be negative, then z must be positive.
- Average endowment, \(\overline{z}_0\), for longevity, it follows that \(\overline{\mu}_0=\mu_0\). Hence, \(\overline{z}_0=1\)
- It would be desirable that the paramteres of \(f_x\) be unchanged for any \(x\).
\[\begin{aligned}
f_x(z)=z^{k-1}\lambda_x^k exp(-z\lambda_x)/\Gamma(K)
\end{aligned}\]
- With mean \(\overline{z}_x=k/\lambda_x\) and variance: \[\begin{aligned} \sigma_x^2(z) = \overline{z}^2_x/k = k/\lambda^2 \end{aligned}\]
- The proportionality assumption has implications for \(f_x(z)\)
- ASPD and the relative risk:
- From the variance of the gamma, the average mortality and the definition of \(\overline{s}_x\), we have: \[\begin{aligned} \sigma_x^2(z)&= \overline{z}^2_x/k\\ \overline{\mu}_x&= \overline{z}_x\mu_x\\ \overline{s}_x&= exp\left(-\int_0^x \overline{\mu}_t dt\right)\\ nq_x(z) &= 1-exp\left(\frac{kz}{\overline{s}_x^{1/k}}-\frac{kz}{\overline{s}_{x+n}^{1/k}}\right)\\ \overline{r}_x&= \frac{\mu_{x1}}{\mu_{x2}}\left(\frac{\overline{s}_{x1}}{\overline{s}_{x2}}\right)^{1/k} \end{aligned}\]
10.1.3.4 Parameter k
- Select values of k focus upon the biological rather than statistical
- Biological dimensions underlying longevity are normally distributed at birth.
- Any deviation from an “optimal” biological point will be associated with decreased survival.
- Conditionally on age, mortality will be a quadratic function
- Each individual’s endowment for longevity (z) is fixed at birth.
- The value of к is the result of n, number of dimensions relevant to longevity.
- The relation of n to the gamma shape parameter is simply n=2k.
- Lower n, the greater is the heterogeneity (higher variance of gamma)
- The values of n used are 1 and 2, suggesting that longevity is unidimensional (k=0.5) and bidimensional (k=1), respectively.
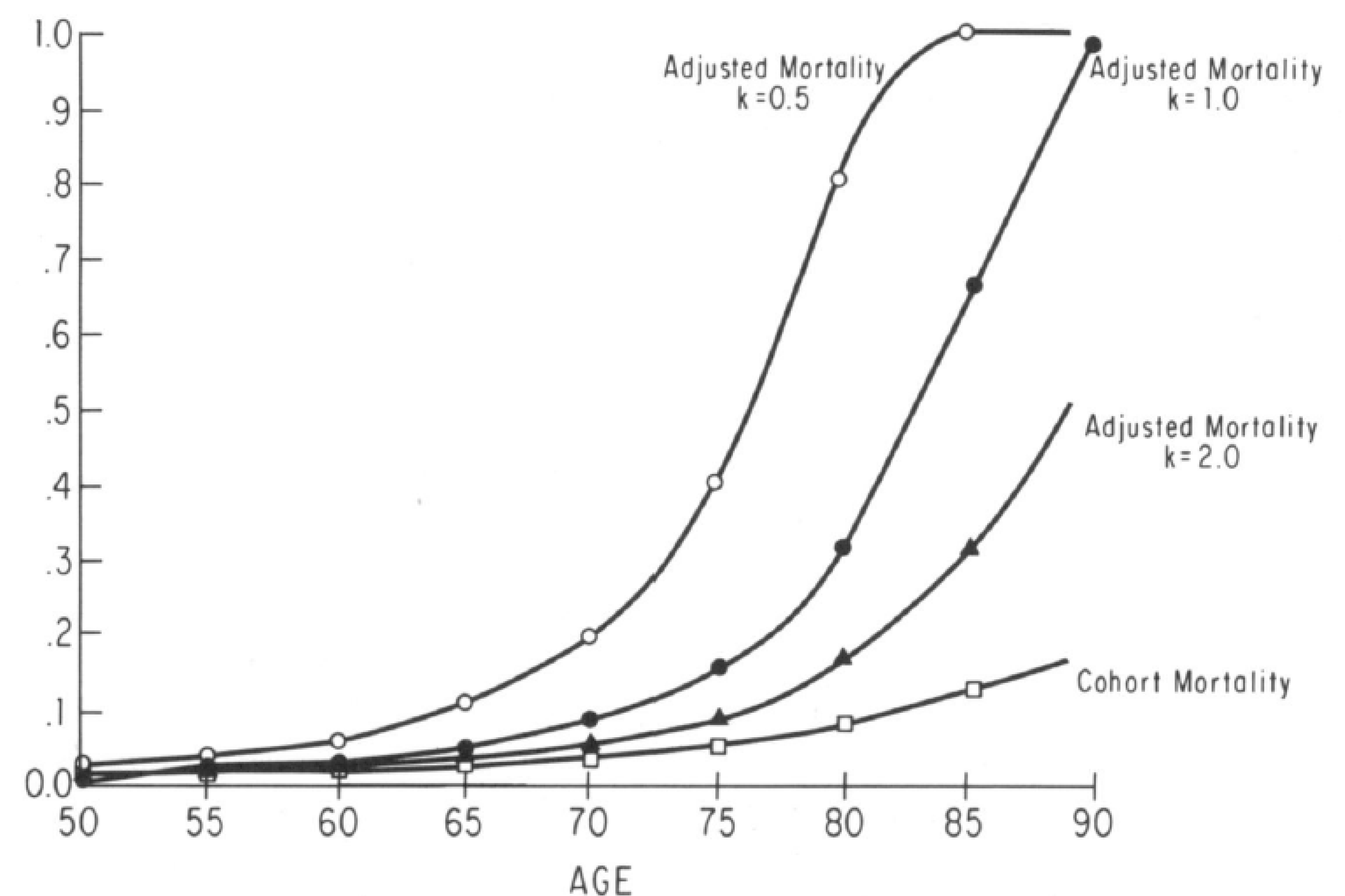
Figure 10.4: Cohort and Individual Age Specific Mortality Probabilities for the 1875 White Female Birth Cohort. Source: Manton and Stallard (1981)
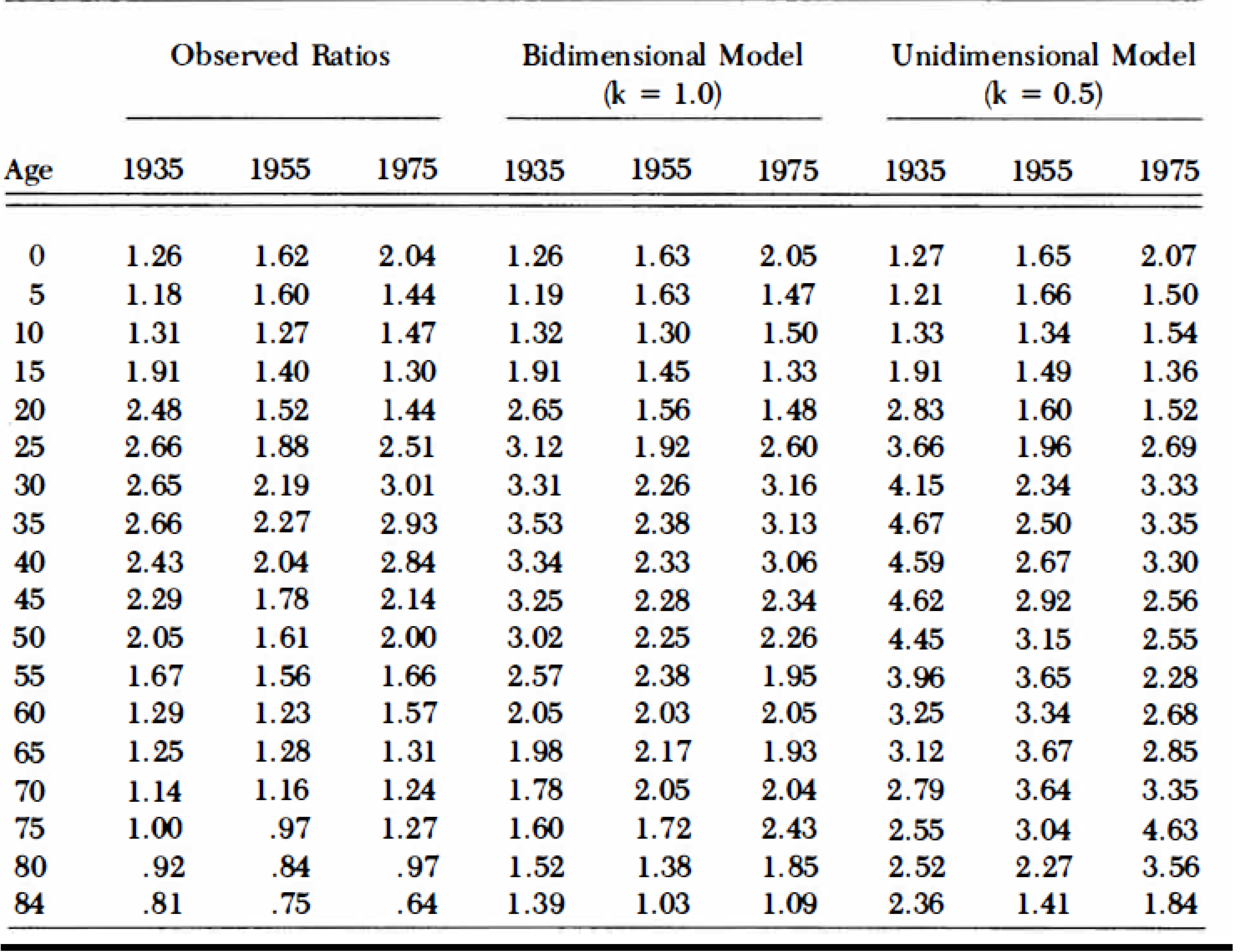
Figure 10.5: Age-specific mortality risk ratios (Black males vs White males) for the years 1935,1955 and 1975. Source: Manton and Stallard (1981)
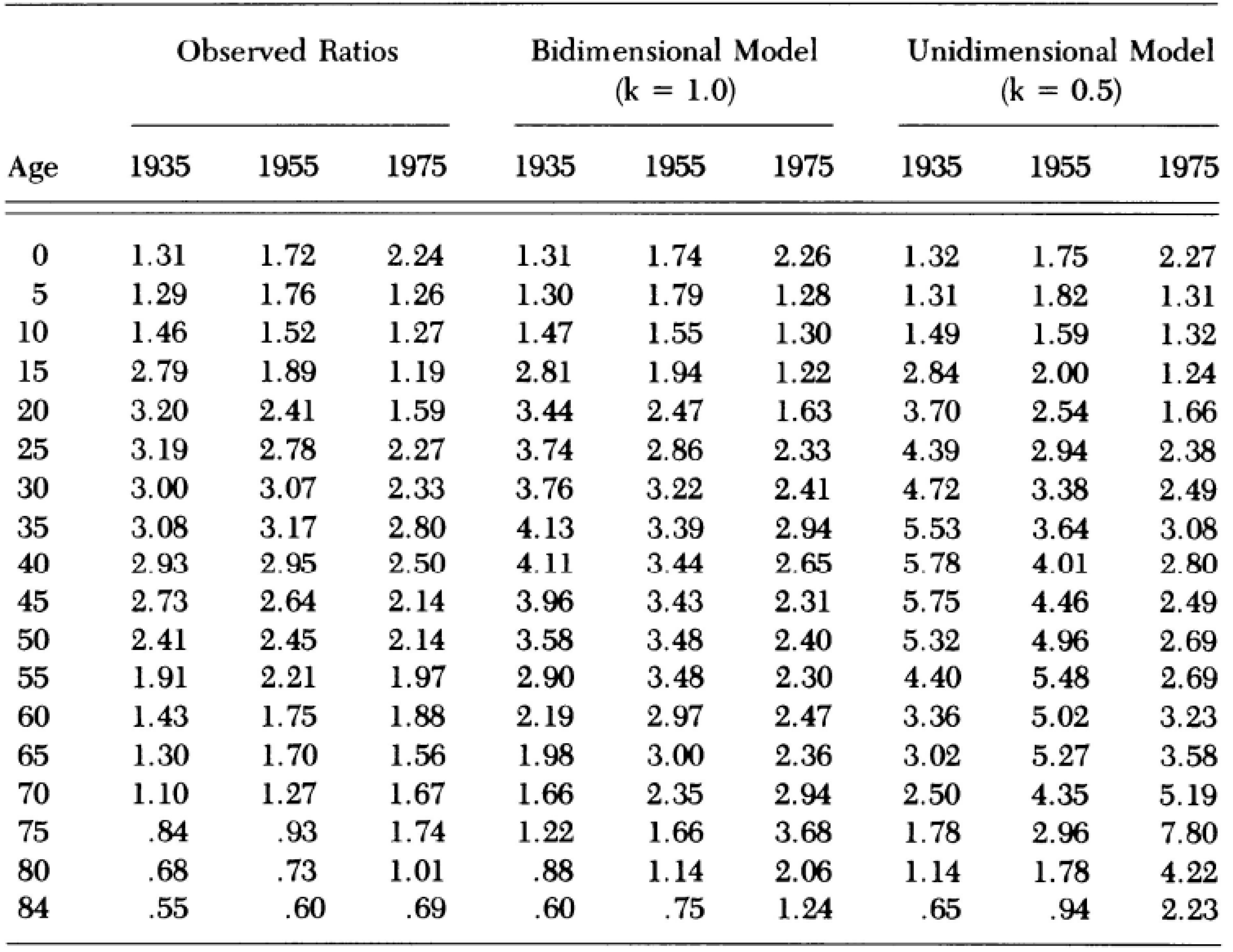
Figure 10.6: Age-specific mortality risk ratios (Black females vs White females) for the years 1935,1955 and 1975. Source: Manton and Stallard (1981)
10.1.3.5 Takeaways
- It seems that the crossover at advanced ages for males is an artifact of the early differential mortality selection.
- An explanation for this differentials at older ages is the relatively more rapid reduction in individual white male mortality
- For black females we can see that they were worse off than males between 25-45 (the childbearing years) at 1935.
- Different Zs will result in a divergence between the increase with age of the cohort mortality rates and the age increase in the probabilities of death for individuals within the cohort.
- This because the earlier selection of the less “robust” population members, implies that individuals age “faster” than their cohorts.
10.1.4 CenSoc Mortality Crossovers
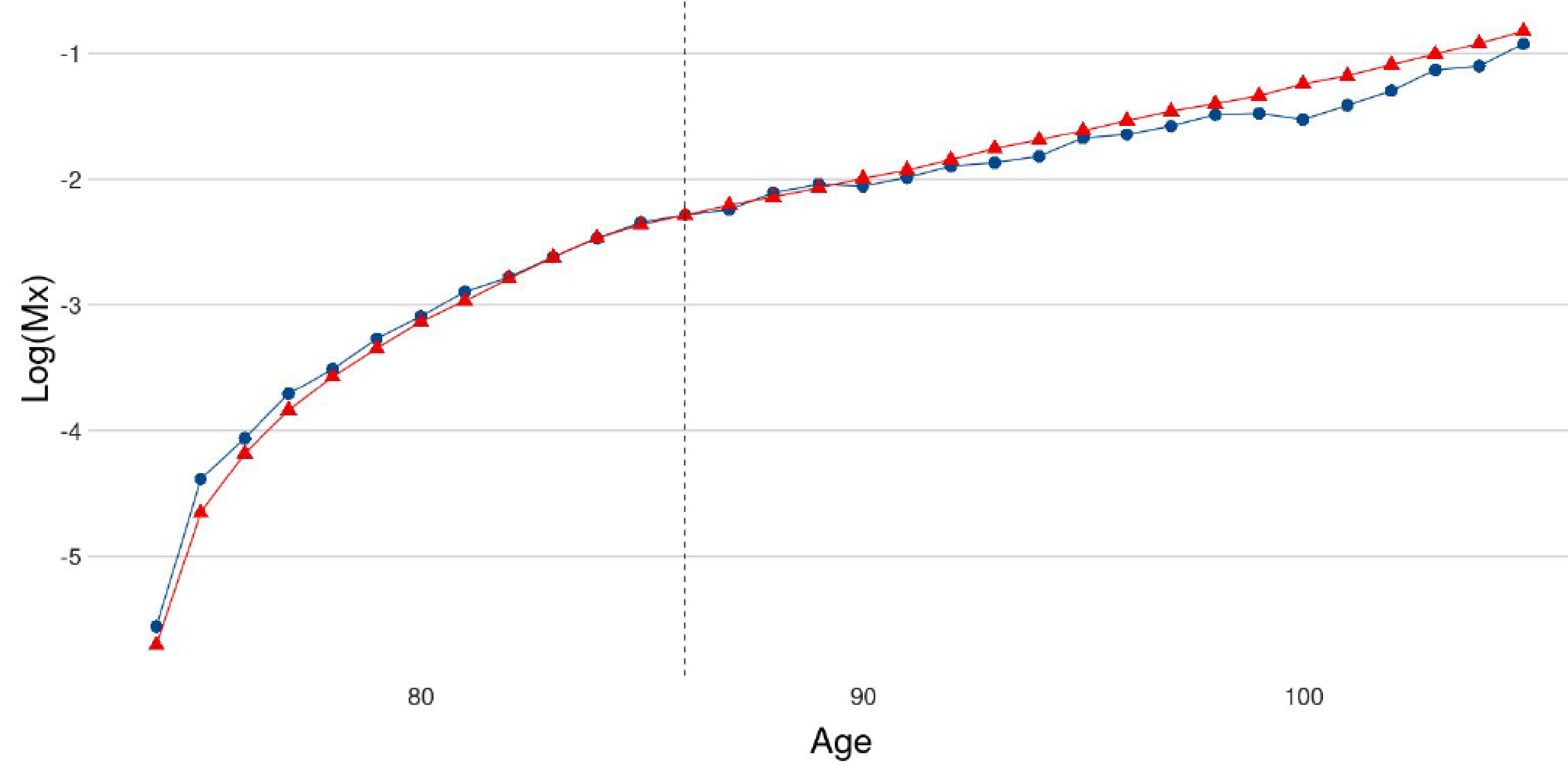
Figure 10.7: Pooled cohorts of 1890-1900
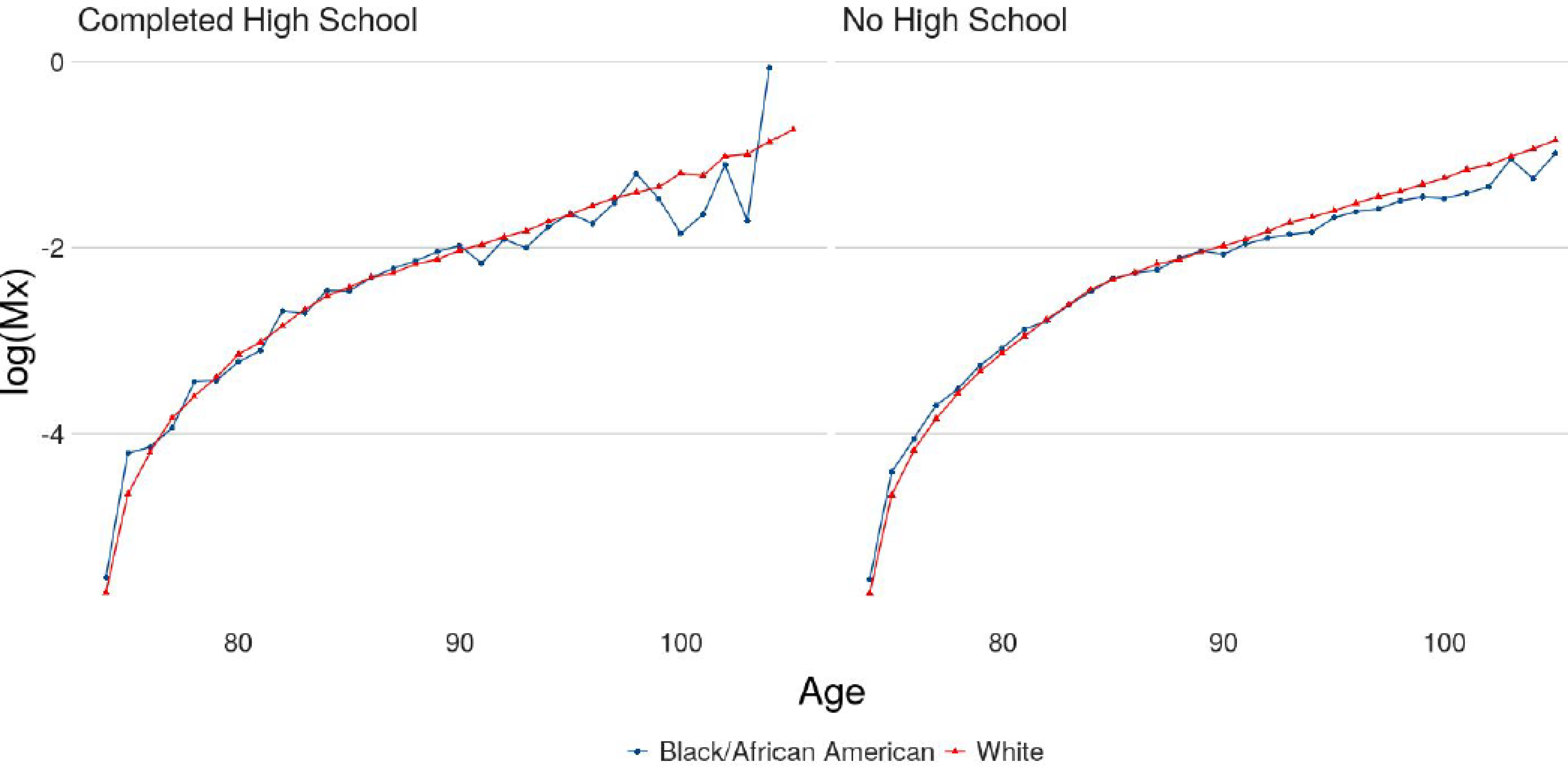
Figure 10.8: Pooled cohorts of 1890-1900, by education level
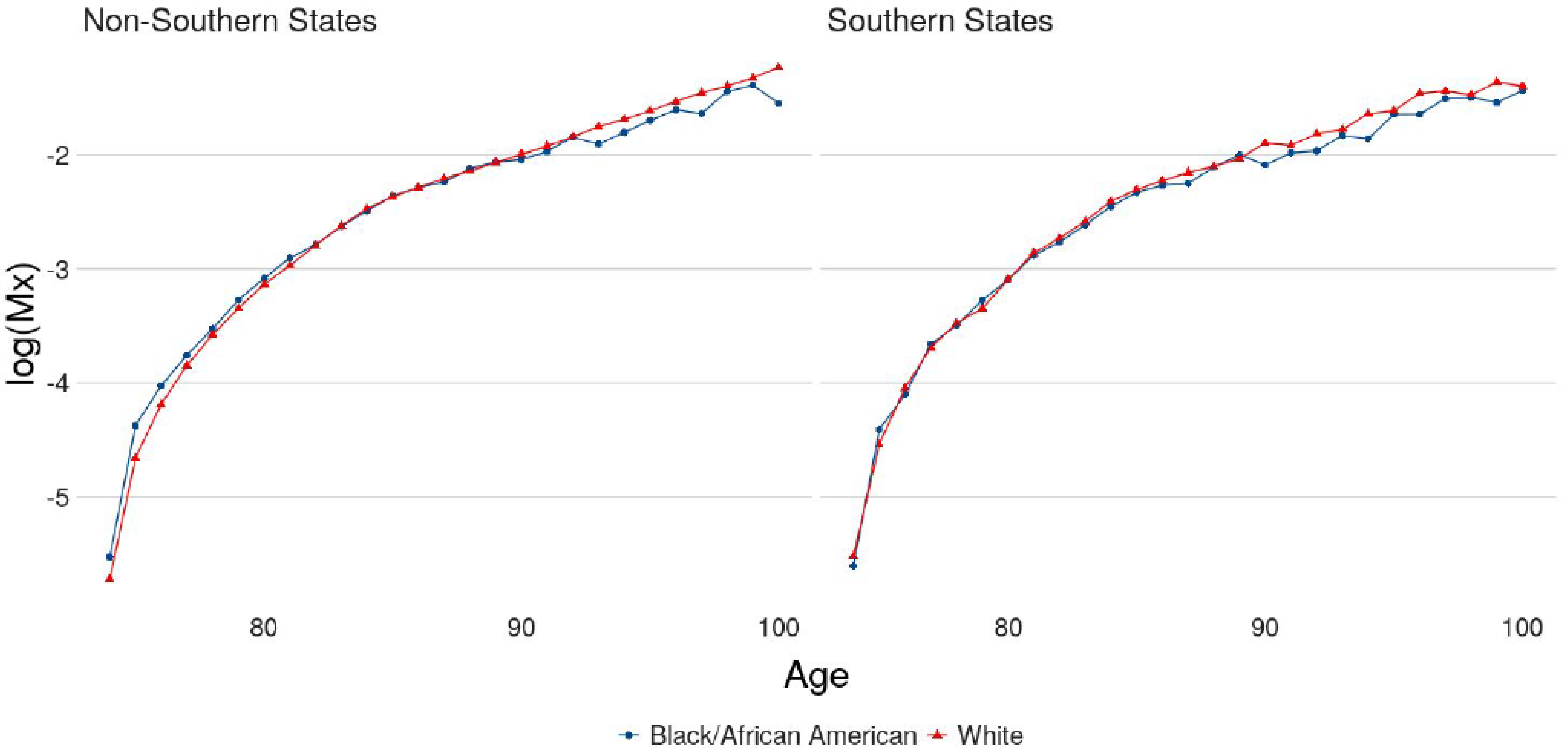
Figure 10.9: Pooled cohorts of 1890-1900 by location
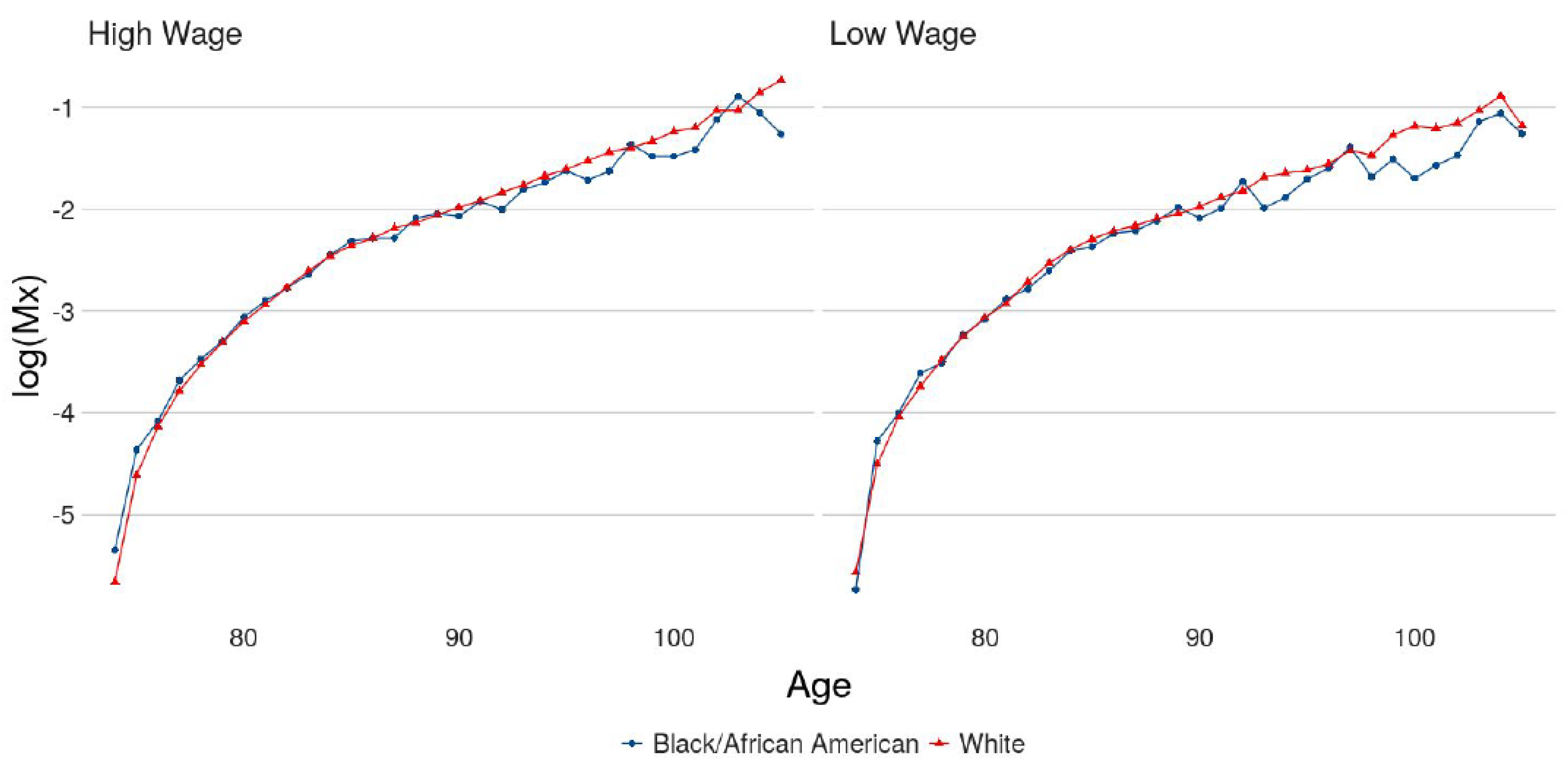
Figure 10.10: Pooled cohorts of 1890-1900 by wages
10.1.4.1 Can the crossover be eliminated?
The baseline hazard is given by \(\mu_{0}(x)=\bar{\mu}(x)e^{\sigma^{2}\bar{H}(x)}\) and we try different values of \(\sigma^2\) for each group of people, in this case Black and White.
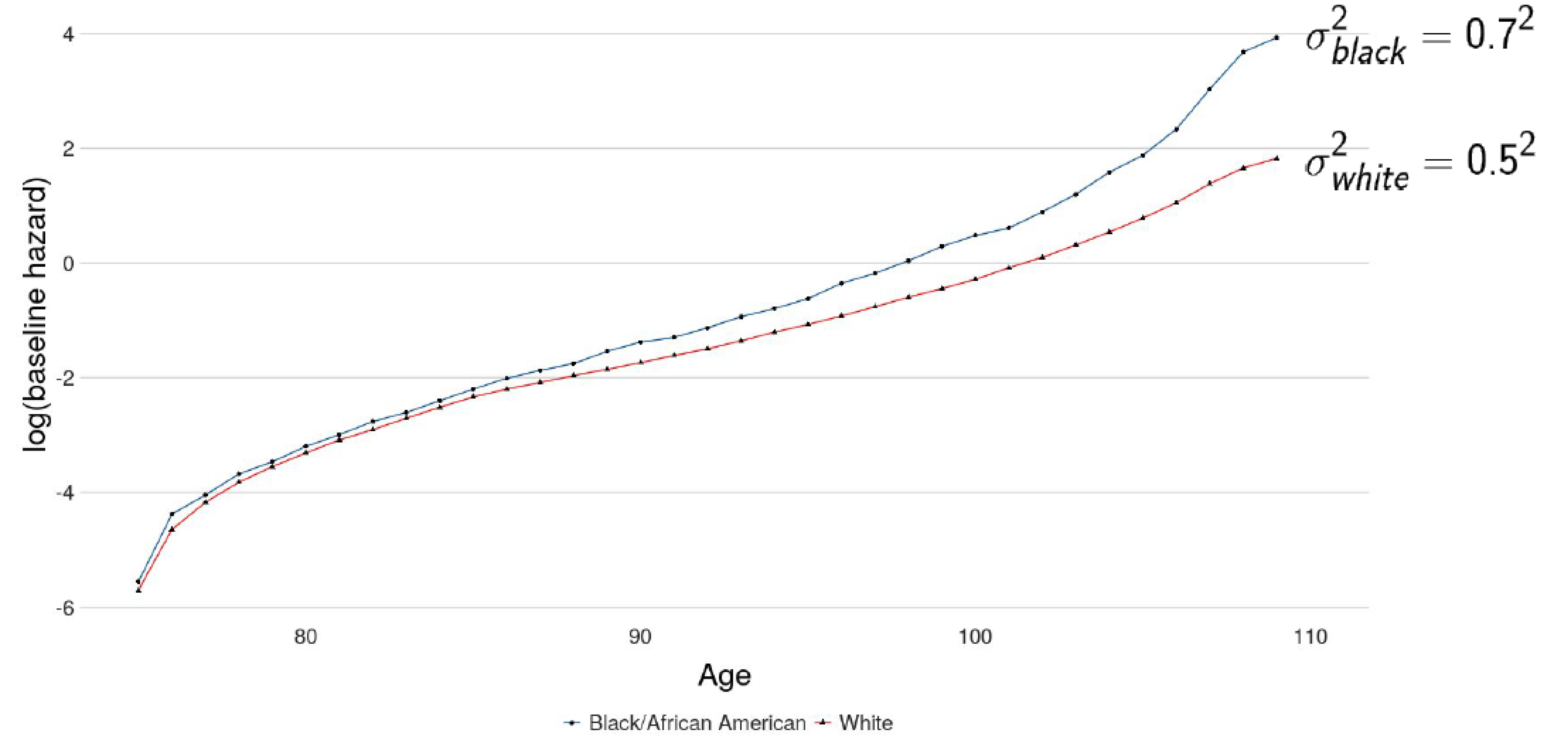
Figure 10.11: Pooled cohorts of 1890-1900 by wages
10.1.4.2 Is death data in CenSoc file less reliable for Blacks?
- 57% of black people in pooled 1890-1900 cohort are missing death
days (day of the month)
- 51% of white people missing death days
- No missing death months
- Missing day of birth far less common (about 0.005% of records)
- Are missing dates indicative of poor data?
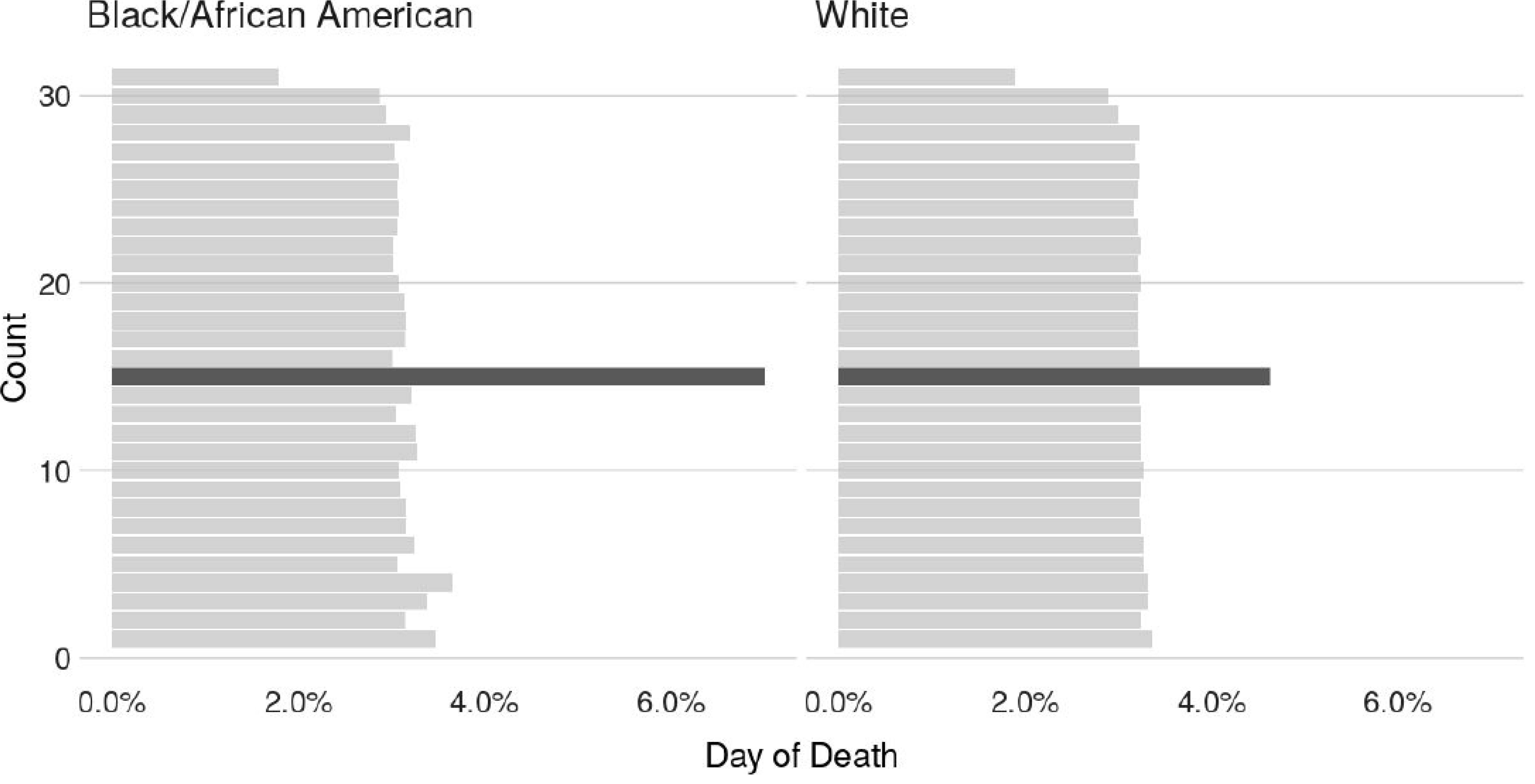
Figure 10.12: Day of death
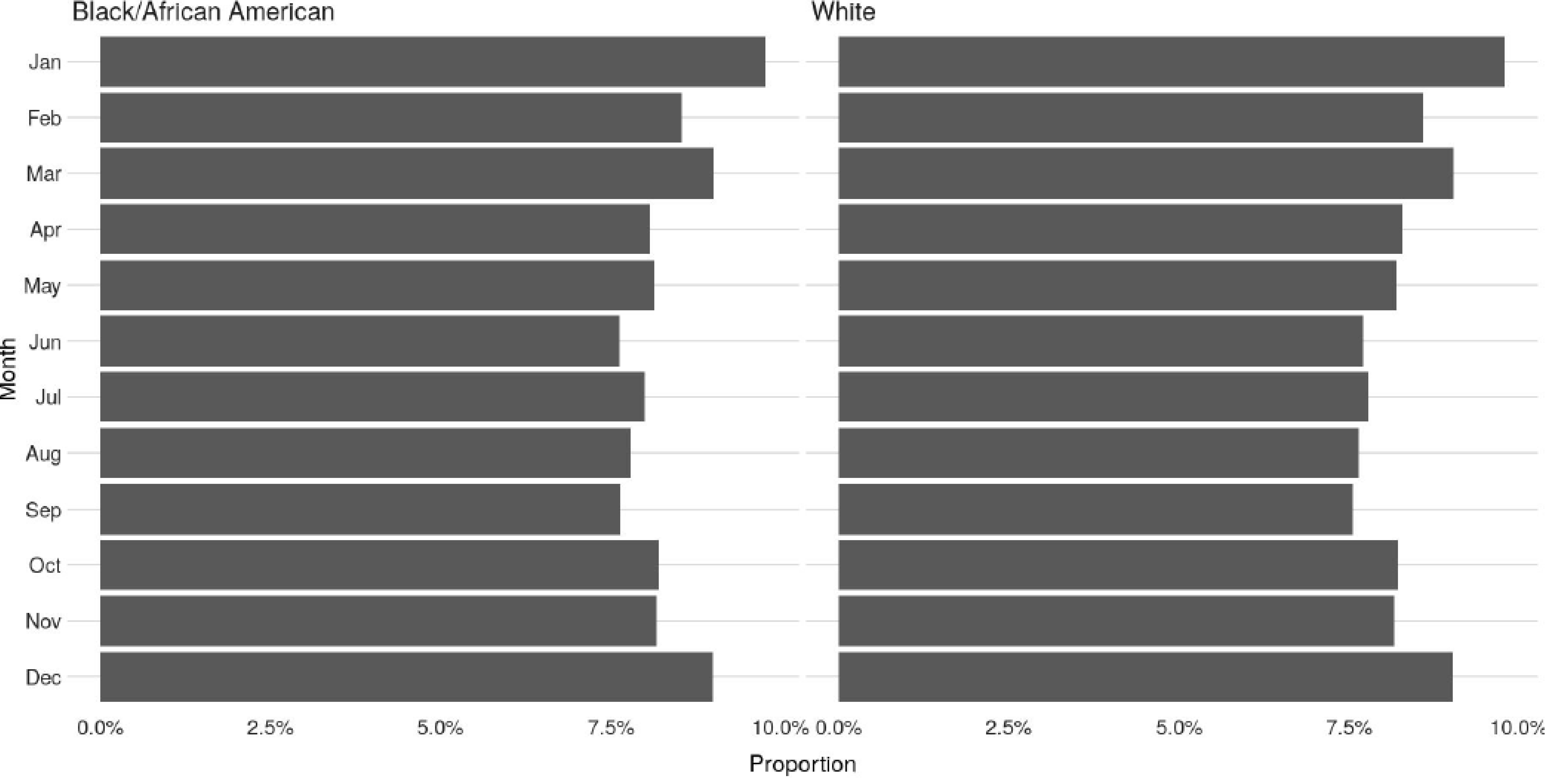
Figure 10.13: Month of death
If observations with missing dates are dropped then the mortality rates look slightly different.
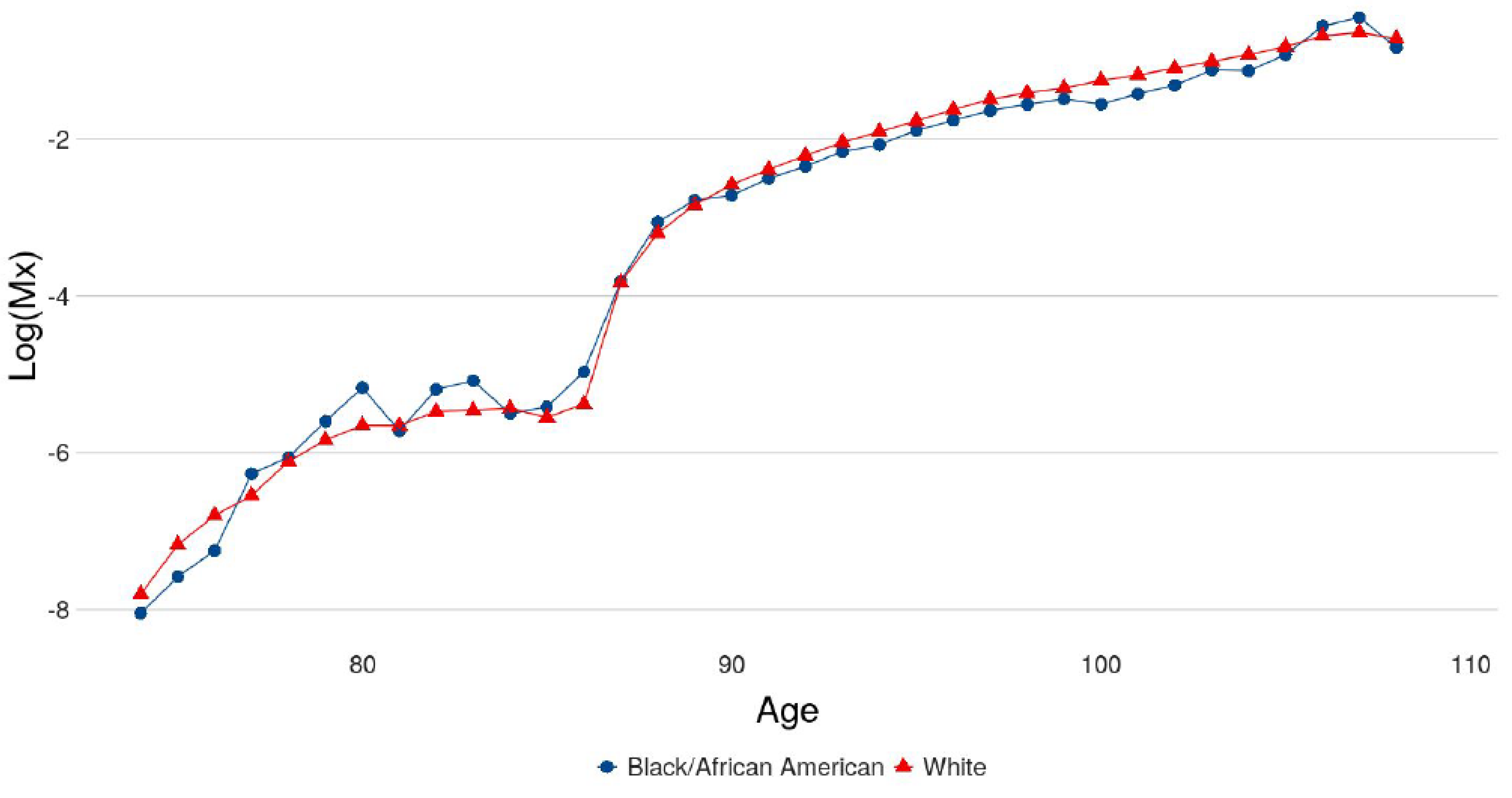
Figure 10.14: Mortality crossover for pooled cohorts of 1890-1900.
10.1.4.3 Heaping for reported birth year
- No Heaping on Death Year
- No Heaping on Age of Death
- Heaping on Birth Year
\(Heaping(1900)=\frac{B_{1900}}{(B_{1899}+B_{1901})/2}\)
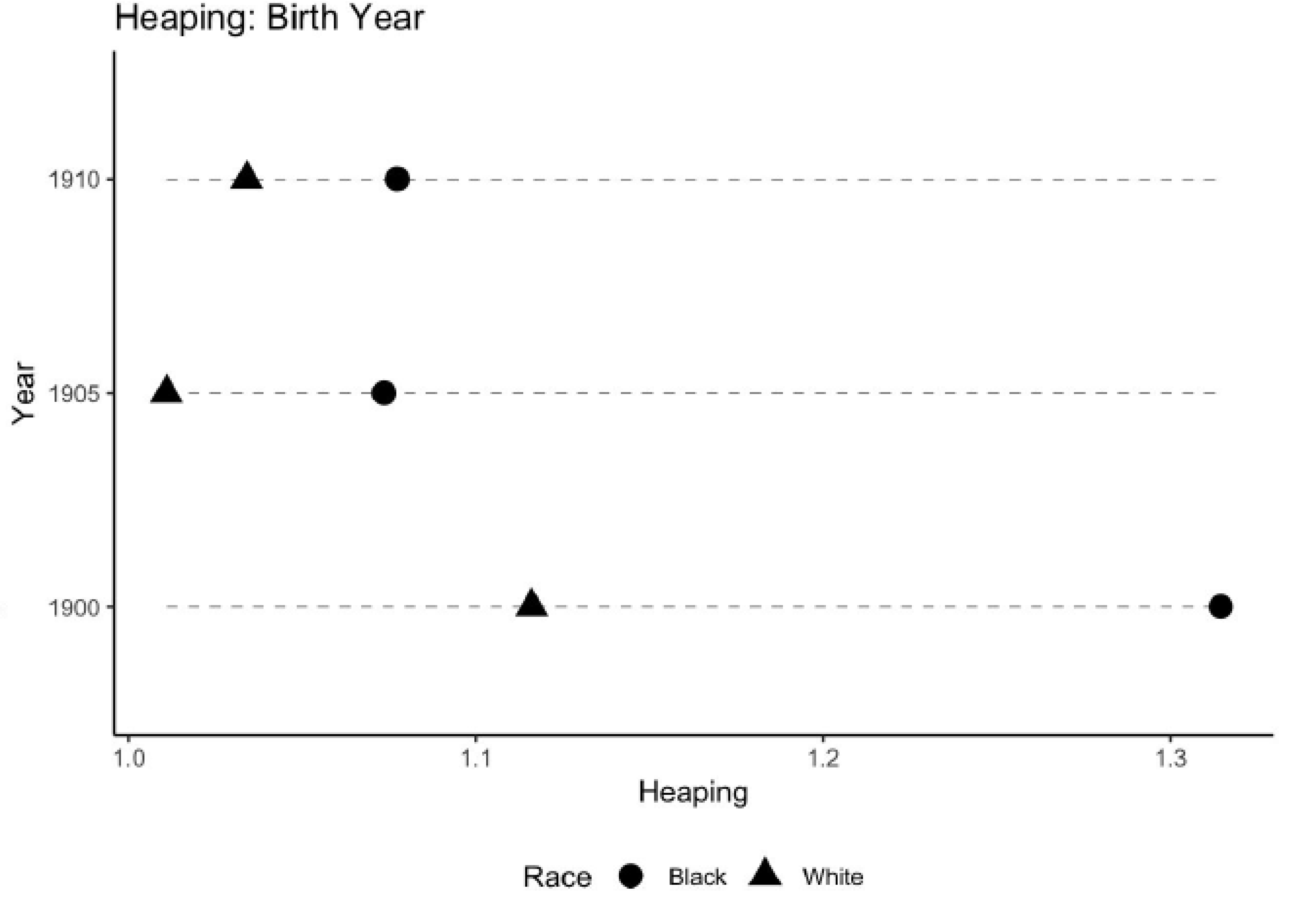
Figure 10.15: Heaping: birth year
10.2 Plateaus
- Steinsaltz and Wachter (2006)
- Barbi et al. (2018): Recent paper claiming to be “the best evidence to date for the existence of extreme-age mortality plateaus in humans”
Using total mortality rates for italian cohorts from 1900 to 1926 (from HMD), we can look at mortality rates over ages and cohorts, as an introduction to Barbi et al. (2018). When focusing on mortality rates above age 60, we see a constantly increasing curve with some variation at very old ages (beyond 100). One important limitation of our life tables is that the rates are only observed up until age 109.
library(tidyverse)
it <- read_table("data/ITA.bltcoh_1x1.txt", skip=1)
it$Age <- as.numeric(it$Age)
it$mx <- as.numeric(it$mx)
it$qx <- as.numeric(it$qx)
it <- it %>% mutate(mx = if_else(mx == 0, as.numeric(NA), mx))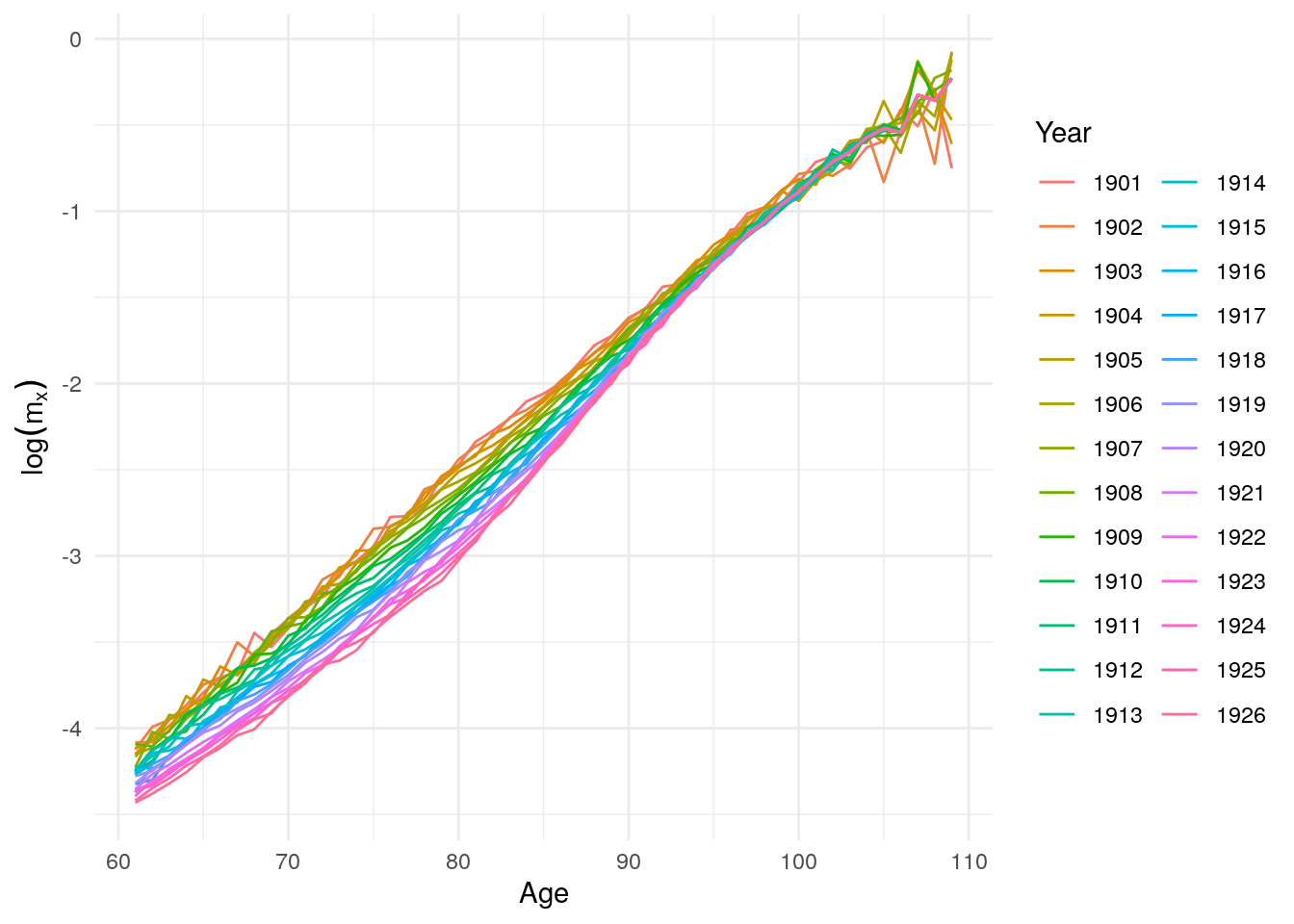
Figure 10.16: Log mortality rates above age 60 after 1900
To detect plateaus above age 105 like the paper, we estimate a Gompertz on ages 60- 100 (there’s some noise from the war) after 1900. Recall that to estimate mortality using hazard rates for Gompertz: \begin{aligned} h(x) = a e^{bx} \ log(h{_x}) = log(a) + bx \end{aligned*} Since we don’t have \(h(x)\) from the lifetable, we use \({_1}m_x\)
min_gomp_age <- 60
max_gomp_age <- 105
max_plateau_age <- 109
min_year <- 1900
gomp <- lm(log(mx) ~ Age, data = it %>% filter(Age > min_gomp_age & Age < max_gomp_age & Year > min_year))
new <- data.frame(Age = max_gomp_age:max_plateau_age)
new$Gomp.pred <- exp(predict(gomp, new))
new <- new %>% left_join(it %>% filter(Age > max_gomp_age-1, Year > min_year) %>% select(Age, Year,mx) %>% spread(Year, mx))
new <- new %>% gather("Year", "mx", -Age)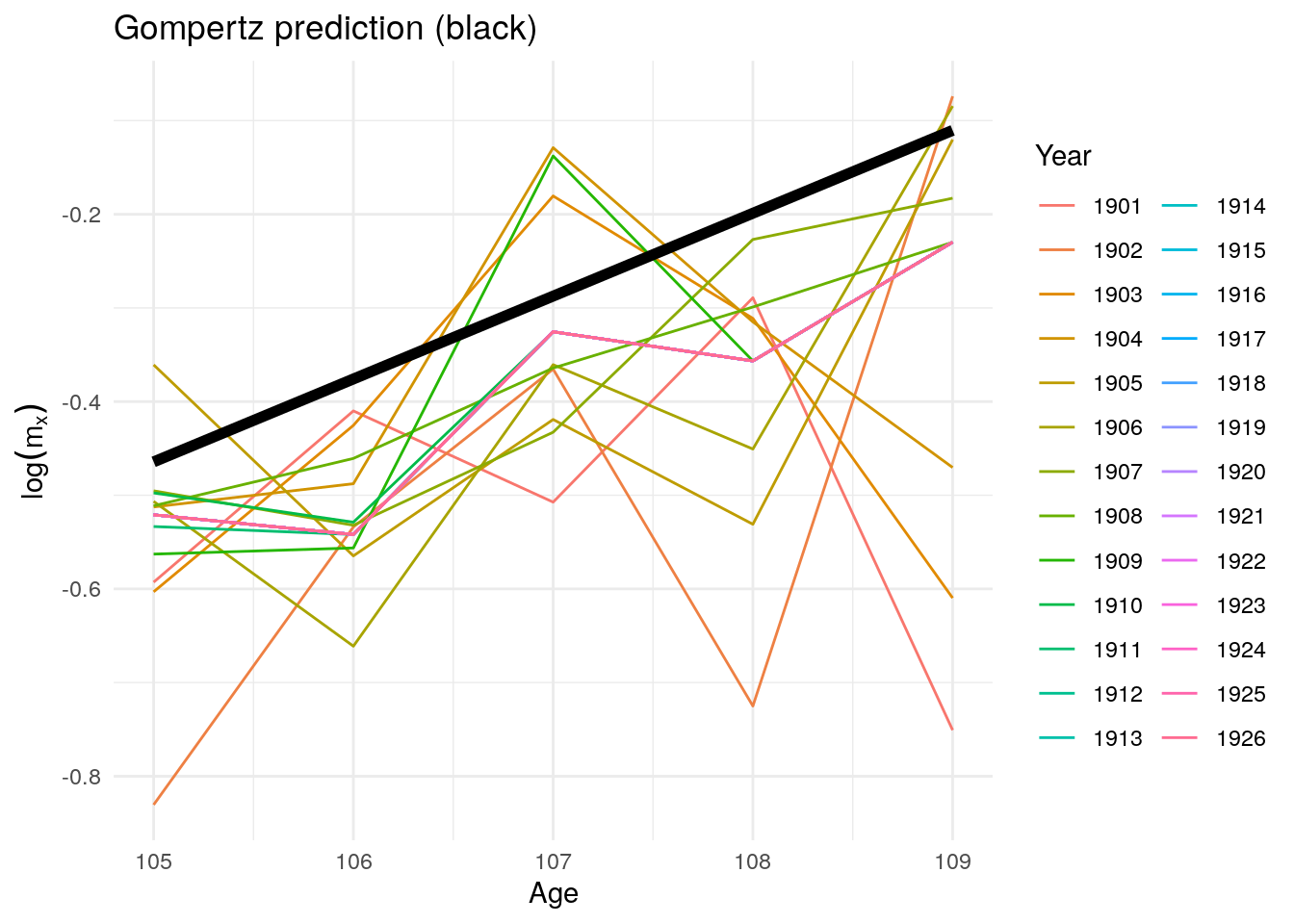
Figure 10.17: Predicted log mortality rates above age 104 after 1900
Now, we look at predicted mortality rates by gender. The graphs below show a similar trend as the previous graph for overall mortality rates. Although the predictions seem to fit the data, that is Gompertz is an adequate method to model mortality between the ages of 100 to 109, a plateau may appear for even later ages. Since HMD data uses ages up to 109, we are unable to observe a plateau as in Barbi et al. (2018).
# For men:
it_m <- read_table("data/ITA.mltcoh_1x1.txt", skip=1)
it_m$Age <- as.numeric(it_m$Age)
it_m$mx <- as.numeric(it_m$mx)
it_m$qx <- as.numeric(it_m$qx)
it_m <- it_m %>% mutate(mx = if_else(mx == 0, as.numeric(NA), mx))
gomp <- lm(log(mx) ~ Age, data = it_m %>% filter(Age > 60 & Age < 100 & Year > 1900))
new <- data.frame(Age = 101:109)
new$Gomp.pred <- exp(predict(gomp, new))
new <- new %>% left_join(it_m %>% filter(Age > 95, Year > 1900) %>% select(Age, Year,mx) %>% spread(Year, mx))
new <- new %>% gather("Year", "mx", -Age)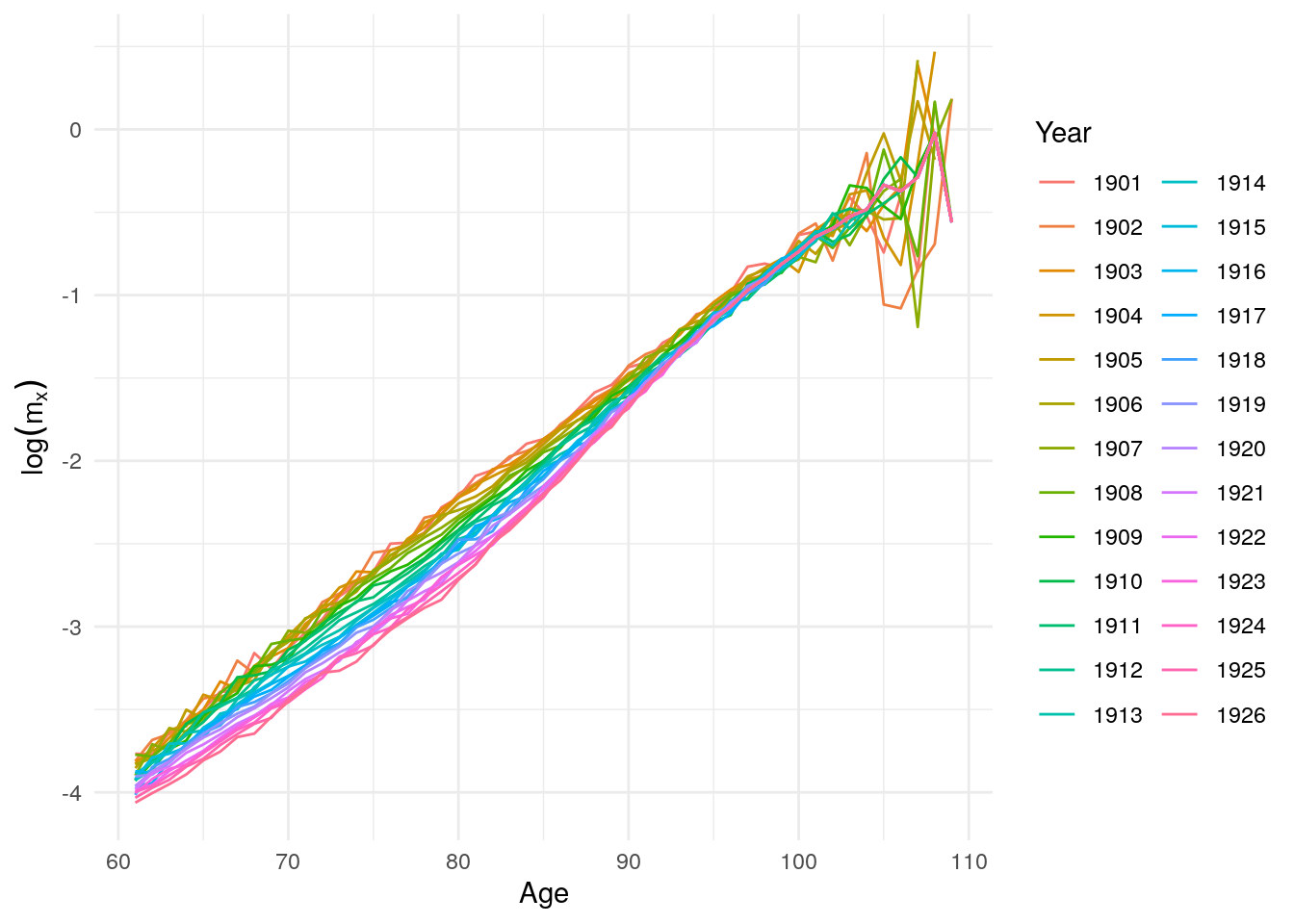
Figure 10.18: Log mortality rates for men above age 60 after 1900
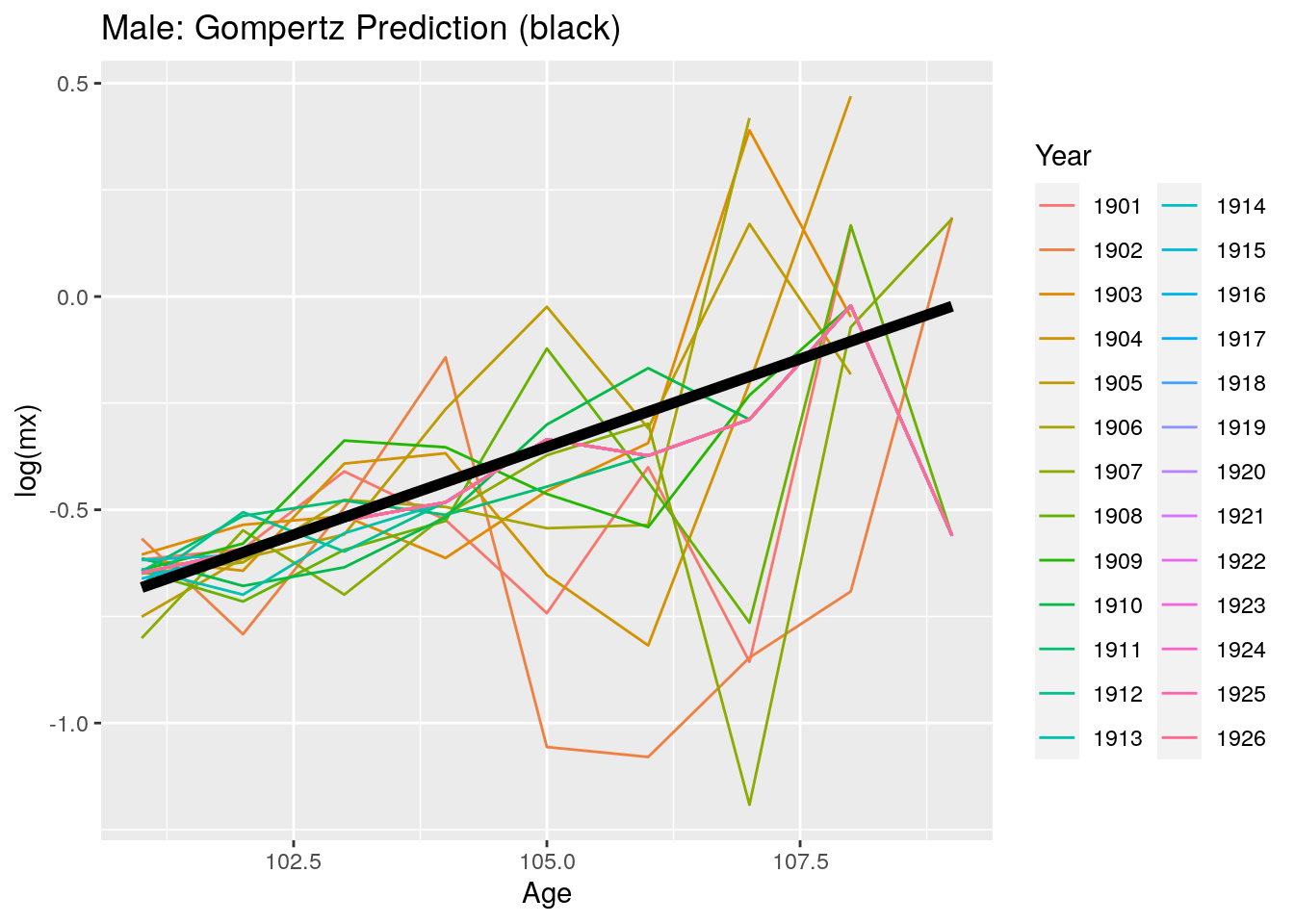
Figure 10.19: Predicted log mortality rates for men above age 99 after 1900
# Women
it_f <- read_table("data/ITA.fltcoh_1x1.txt", skip=1)
it_f$Age <- as.numeric(it_f$Age)
it_f$mx <- as.numeric(it_f$mx)
it_f$qx <- as.numeric(it_f$qx)
it_f <- it_f %>% mutate(mx = if_else(mx == 0, as.numeric(NA), mx))
gomp <- lm(log(mx) ~ Age, data = it_f %>% filter(Age > 45 & Age < 100 & Year > 1900))
new <- data.frame(Age = 101:109)
new$Gomp.pred <- exp(predict(gomp, new))
new <- new %>% left_join(it_f %>% filter(Age > 95, Year > 1900) %>% select(Age, Year,mx) %>% spread(Year, mx))
new <- new %>% gather("Year", "mx", -Age)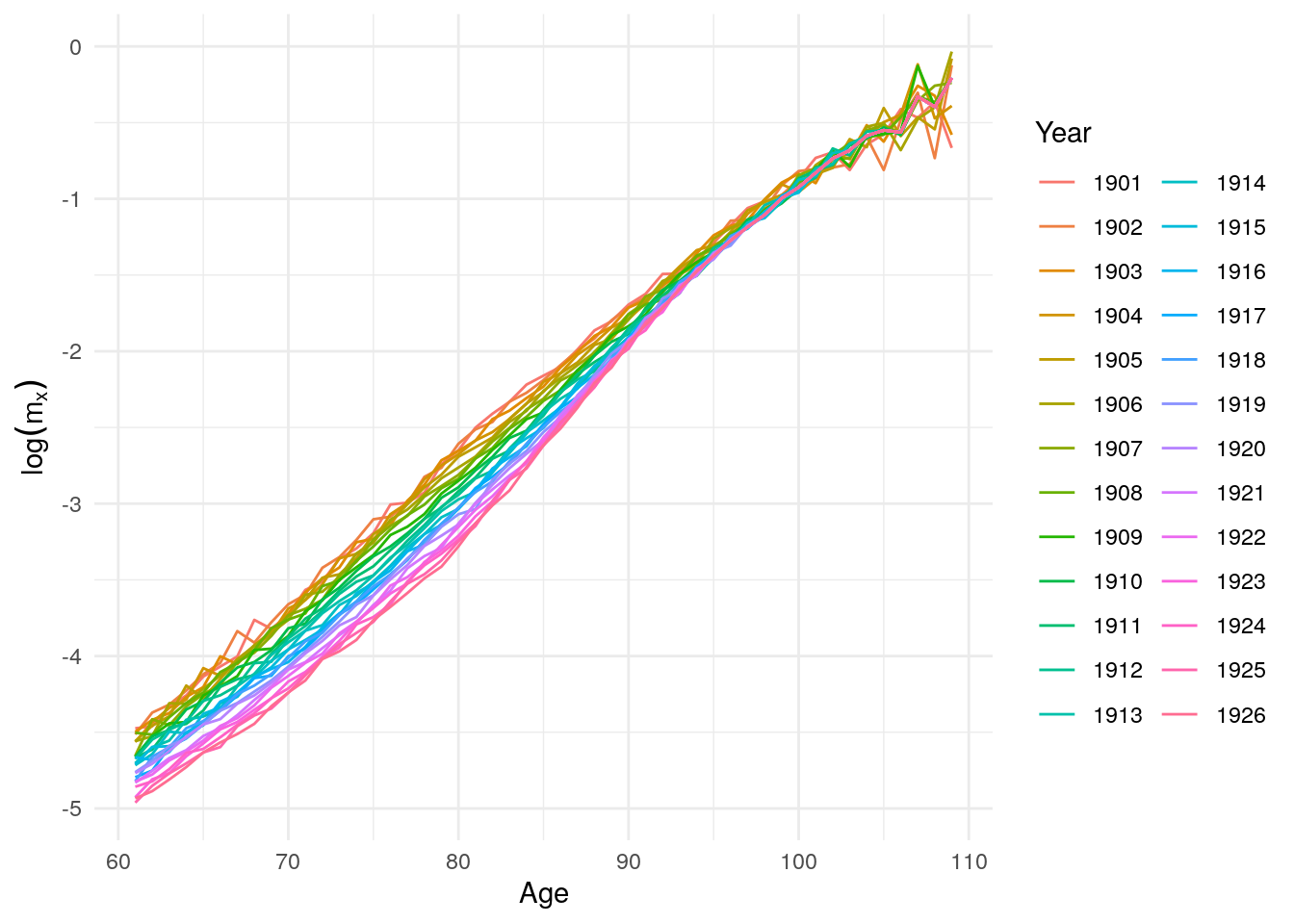
Figure 10.20: Log mortality rates for women above age 60 after 1900
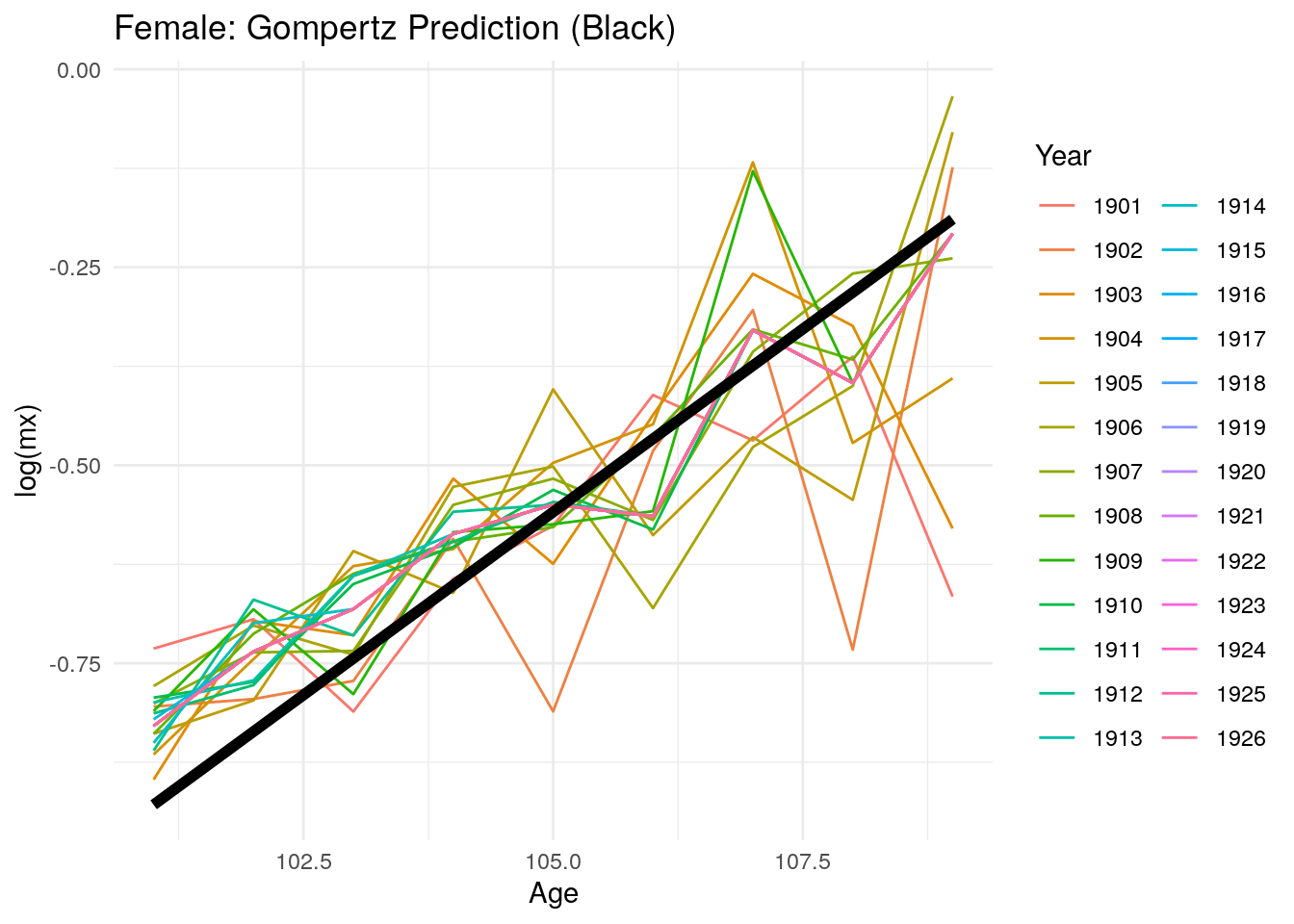
Figure 10.21: Predicted log mortality rates for women above age 99 after 1900
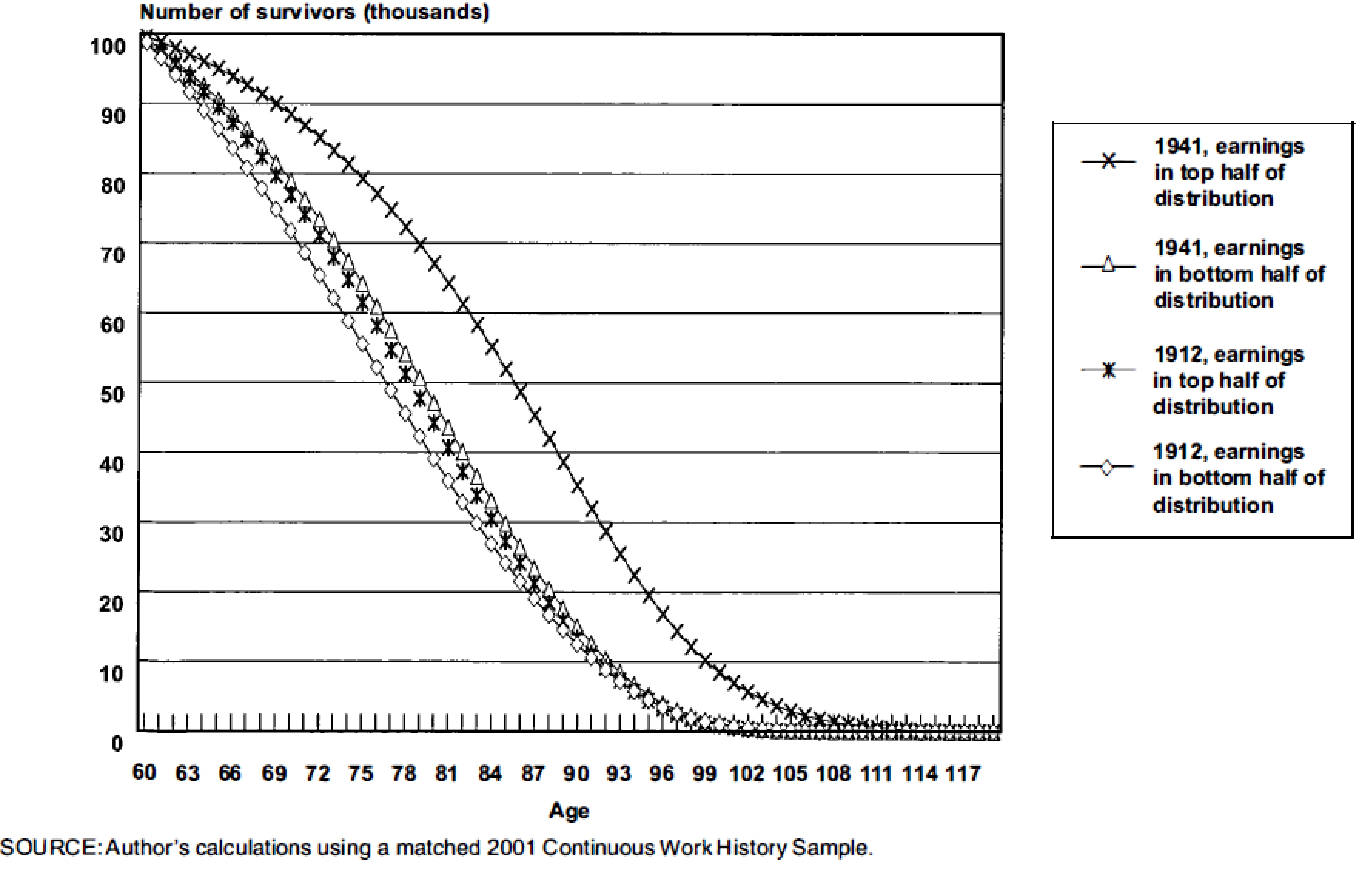
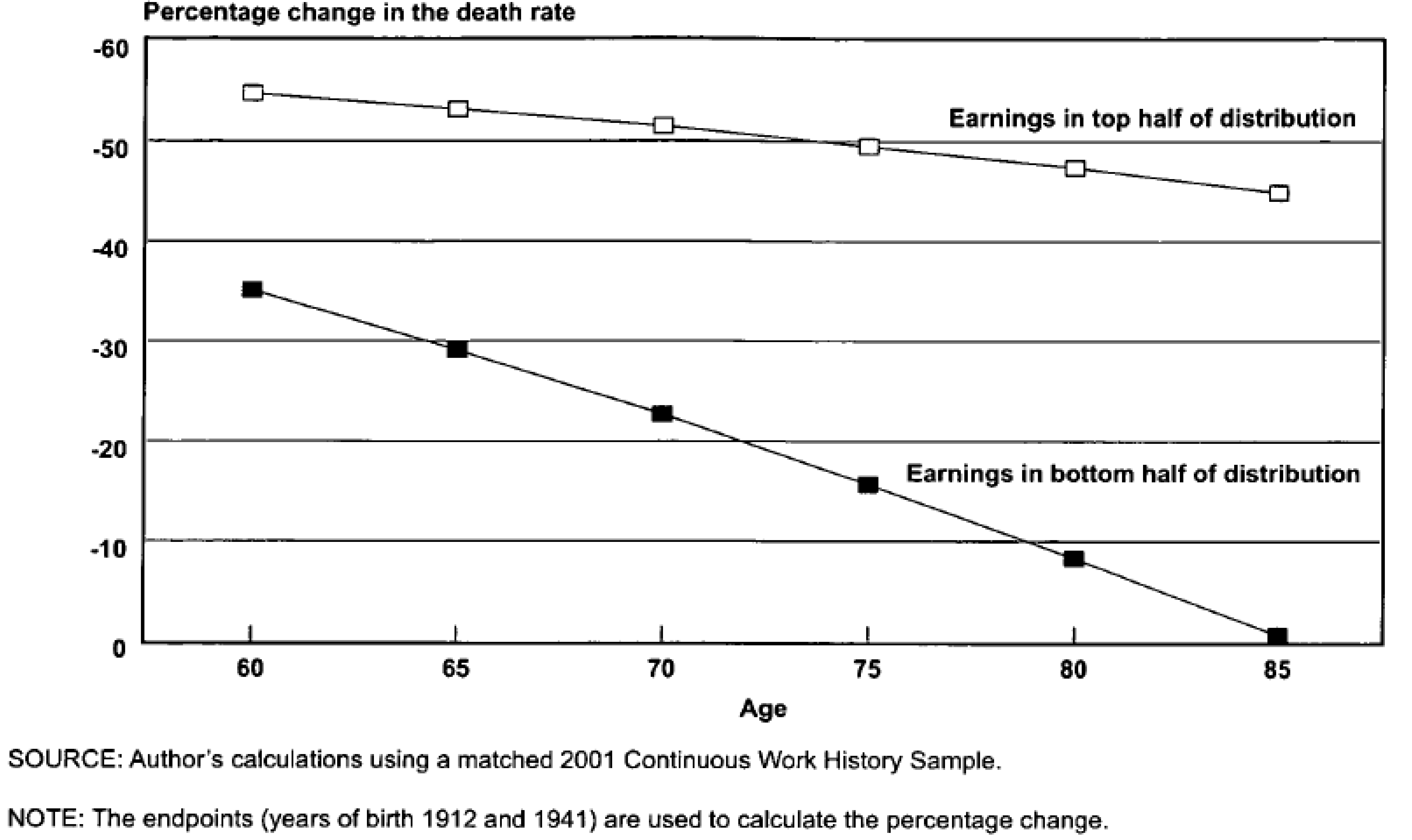
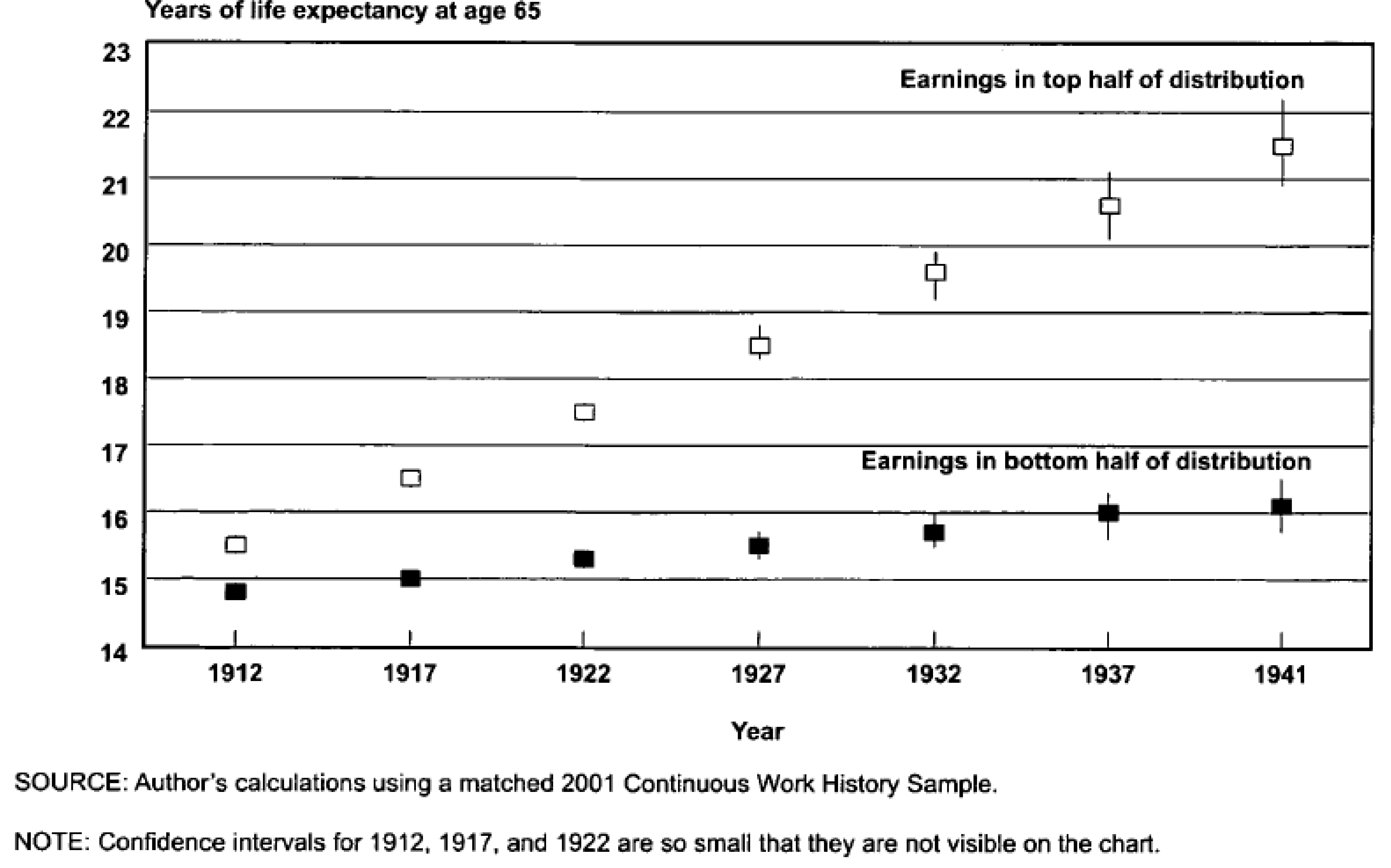
10.3 Rising Inequality
- Waldron (2007): Age-cohort model suggesting growing inequality by income, but includes many caveats about heterogeneity. Our challenge is to apply models of heterogeneity to this issue, particularly the result on mortality improvement over time.
References
Barbi, Elisabetta, Francesco Lagona, Marco Marsili, James W Vaupel, and Kenneth W Wachter. 2018. “The Plateau of Human Mortality: Demography of Longevity Pioneers.” Science 360 (6396): 1459–61.
Coale, Ansley J, and Ellen Eliason Kisker. 1986. “Mortality Crossovers: Reality or Bad Data?” Population Studies 40 (3): 389–401.
Manton, Kenneth G, and Eric Stallard. 1981. “Methods for Evaluating the Heterogeneity of Aging Processes in Human Populations Using Vital Statistics Data: Explaining the Black/White Mortality Crossover by a Model of Mortality Selection.” Human Biology, 47–67.
Steinsaltz, David R, and Kenneth W Wachter. 2006. “Understanding Mortality Rate Deceleration and Heterogeneity.” Mathematical Population Studies 13 (1): 19–37.
Waldron, Hilary. 2007. “Trends in Mortality Differentials and Life Expectancy for Male Social Security-Covered Workers, by Socioeconomic Status.” Soc. Sec. Bull. 67: 1.